
Inherited Retinal Disease Management: Brian Strem from Kiora Pharmaceuticals in a Stimulating Dialogue Exchange with PharmaShots
Shots:
-
Recently, Kiora Pharmaceuticals received an upfront payment of $16M after inking the partnership agreement with Théa Open Innovation to develop and commercialize KIO-301 and is eligible to receive up to $285M
-
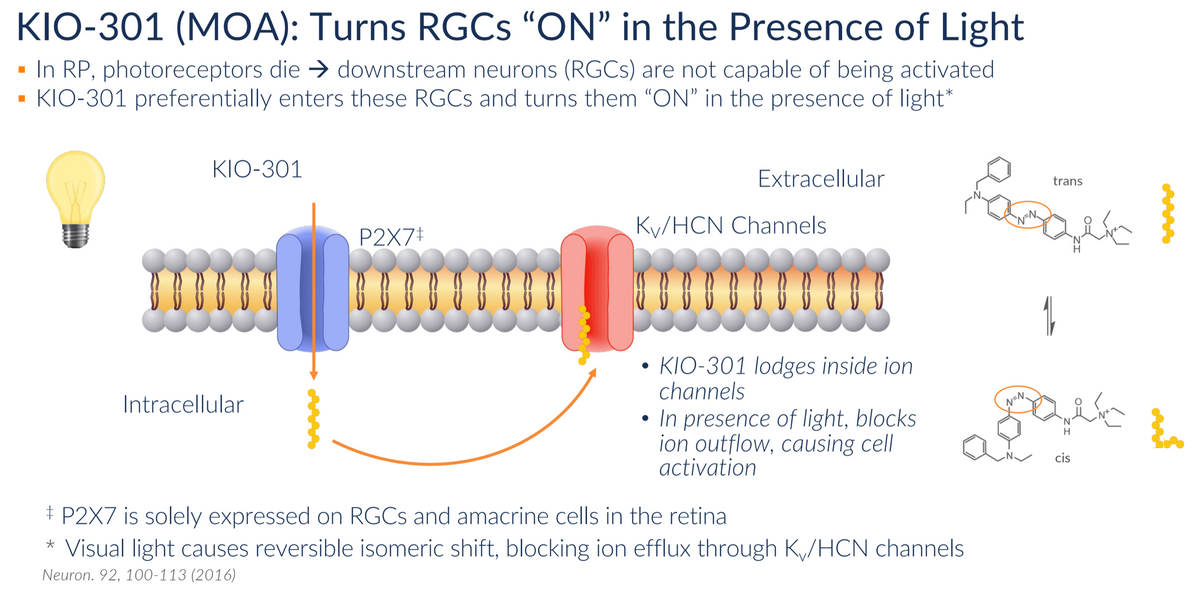
KIO-301 belongs to a new-generation drug called molecular switches that offer a path around damaged receptors rather than looking to repair gene mutation
-
Today, at PharmaShots, we have Brian Strem shedding light on KIO-301
Saurabh: Kudos for bringing up clinical trial results to light- paving the way for the development of the breakthrough drug for the treatment of Retinal disorders. First, Congratulations on your partnership with Théa Open Innovation (TOI). Can you tell us about this strategic partnership and how will this partnership impact the development of KIO-301?
Brian: We’re very excited about what this partnership holds for both us and Théa. To break it down, Théa will have co-development and commercialization rights for KIO-301 while Kiora receives a $16M upfront payment with the possibility of an additional $285M in clinical development, regulatory and commercial milestone payments. However, the impact of this deal is so much greater than just financial assets. The strategic guidance from Théa in advancing KIO-301 to market is a huge win for us, as they have the commercial resources to ensure that this therapy benefits as many patients as possible.
Saurabh: Can you brief us about KIO-301 and its MOA?
Brian: KIO-301 is a small molecule photoswitch that has the ability to help restore some vision in individuals with inherited retinal diseases in a gene mutation-agnostic manner. This means, irrespective of the gene mutation underlying a patient’s progression, the drug has the potential to help. It has the ability to get inside neurons downstream of dead photoreceptor cells in the eye and relay light signals, which was previously (and in healthy individuals) the role of the photoreceptors.
Saurabh: What makes this asset unique?
Brian: This is the first dynamic small molecule that demonstrates the ability to restore vision in a patient. This molecule belongs to an entirely new class of drugs called molecular photoswitches, and Kiora is the first to develop it. There are other technologies in clinical trials, like gene therapies that are looking to repair the hundreds of gene mutations that underlie retinitis pigmentosa. However, this means a single gene therapy will only affect a sliver of the diseased population, while molecular photoswitches don’t look to repair a gene mutation, they offer a path around the damaged receptors.
Saurabh: What can we expect in 2024 from the development of KIO-301?
Brian: The Phase 2 clinical trial is expected to initiate later in 2024 and is anticipated to enroll about 36 patients. It will be a randomized, controlled trial where both patients and the treating physicians will be masked. Patients will have the drug injected into both eyes and will return every six weeks for a total of three injections. Since there were no safety concerns observed in the first trial, the team will be conducting additional exploration of the dose to find the highest level of efficacy. Patients included in the study will be those with the most pronounced vision loss, which is the population Kiora hopes and expects to have first access to the treatment upon potential entry to market, assuming the molecule continues to demonstrate both safety and efficacy.
Saurabh: What unmet needs does KIO-301 fulfill in the treatment of Rare Retinal disorders?
Brian: Currently, patients with retinitis pigmentosa have no therapeutic options, they only have lifestyle options. There are no existing routes to stop their oncoming blindness, so they must learn to adjust to life without vision. This includes learning to live with a cane, making changes inside their home, working with an assisted walking dog, learning braille and more. Imagine a world where those patients might still be able to have some level of vision restored so that they could see light or shapes or even just navigate around more independently. That’s the promise KIO-301 holds.
Saurabh: How does Kiora’s focus on orphan indication involve cost-effective paths to market?
Brian: It’s important to impress that a cost-effective path to market was never Kiora’s goal in pursuing treatment for retinitis pigmentosa. We identified an unmet need in finding new treatments for patients with inherited retinal diseases, RP in particular, and it just so happens that these inherited diseases are often rare diseases. This generally comes with an accelerated path to market. While this is a welcomed benefit of working in this space, so that we can see our patients benefit from our therapies faster, it was never the sole reason we began developing KIO-301.
Saurabh: What challenges did the company face during the P-I trial of KIO-301?
Brian: The Phase 1 clinical trial (ABACUS-1) was a first-in-human clinical trial with a novel approach to potentially restore vision in patients with late-stage disease progression due to retinitis pigmentosa. This population historically has had few opportunities to participate in clinical trials as most therapies in development have targeted patients with earlier-stage disease. As such, there was not a significant number of historical tests that Kiora and the physicians and patients participating in the study could use. Further, this was the first clinical trial performed using a molecular photoswitch, so it was important to us to be as methodical as possible with the implementation of the trial so that we would answer essential questions and come out with actionable results. We learned a lot through this process, particularly about setting up the right endpoints. Heading into our Phase 2 trial, we’ve learned how to set up even better functional vision endpoints and continue discussions with regulators to ensure the tests meet their requirements as well. Learning and growing through the drug development process is key with any emerging therapeutic, but especially when conducting clinical trials involving new mechanisms of action.
Image Source: Canva
About the Author:

Brian Strem
Brian Strem, Ph.D., is a biotech executive and entrepreneur with a strong ophthalmology background. Dr. Strem co-founded Bayon Therapeutics, where he in-licensed global, exclusive rights to the Intellectual Property covering multiple generations of photoswitches. He also co-founded Okogen Inc., an ophthalmic company focused on a novel therapeutic for the treatment of viral infections of the eye. Dr. Strem has also held leadership positions at Sound Pharmaceuticals, Allergan, Shire Pharmaceuticals and Cytori Therapeutics. He received his B.S. in bioengineering from Cornell University and his Ph.D. in biomedical engineering from UCLA.
Related Post: Off the Shelf Platform: Efrat Kaduri from Pluri Inc. In a Riveting Conversation with PharmaShots
Tags

Saurabh is a Senior Content Writer at PharmaShots. He is a voracious reader and follows the recent trends and innovations of life science companies diligently. His work at PharmaShots involves writing articles, editing content, and proofreading drafts. He has a knack for writing content that covers the Biotech, MedTech, Pharmaceutical, and Healthcare sectors.














